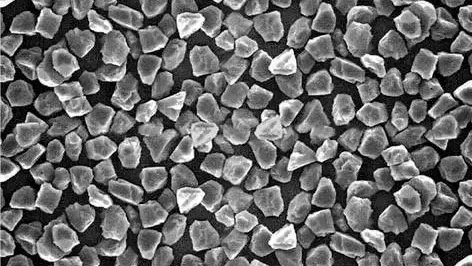
Nanopowders have characteristics that differ from both atoms and crystals. They can be considered a new material, which is different from bulk materials. They have physical and chemical properties that significantly differ from those of bulk materials.
The surface structure of nanoparticles differs from the internal structure. The atomic spacing inside the particles is generally smaller than that in bulk materials, but it can also increase. The electronic energy level structure of nanoparticles are differernt from the bulk solids. This is due to factors such as electrical neutrality and the constraints on electron movement. When the size of small particles enters the nanoscale, the particles and the nanosolids exhibit the following effects:
1. Characteristics of Nanopowder

1)Discontinuity of electronic energy levels
Kubo’s theory: Kubo’s theory addresses the distribution of electronic energy levels near the Fermi surface of metal ultrafine powder particles. This theory differs from traditional approaches. They usually focus on the distribution of electronic energy levels near the Fermi surface of bulk materials. When the particle size reaches the nanometer level, the quasi-continuous energy levels of the original bulk metal become discrete. It’s due to the quantum size effect.
2)Quantum size effect
When the particle size drops below a threshold, the electronic energy levels near the metal Fermi level change from quasi-continuous to discrete. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy levels of nano-semiconductor particles also become discontinuous, leading to an increase in the energy gap. This phenomenon is called the quantum size effect. Band theory indicates that the electronic energy levels near the metal Fermi level are generally continuous.
For nanoparticles, however, the number of atoms they contain is limited. The number of conductive electrons is very small, causing the energy level spacing to split. When the energy level spacing exceeds thermal energy, magnetic energy, static magnetoelectric energy, electrostatic energy, photon energy, or the condensation energy of the superconducting state, the quantum size effect must be considered. This effect results in the magnetic, optical, acoustic, thermal, electrical, and superconducting properties of nanoparticles being significantly different from those of bulk materials.
3)Small size effect
When the size of ultrafine particles is comparable to or smaller than physical characteristics such as the wavelength of light, de Broglie wavelength, and the coherence length or transmission depth of the superconducting state, the boundary conditions of the particle’s periodicity are disrupted. The atomic density near the surface layer of the amorphous nanopowders decreases, resulting in new small size effects in their acoustic, optical, electrical, magnetic, thermal, and mechanical properties.
4)Surface effect
Nanopowders are small in size and have high surface energy, with atoms located on the surface accounting for a significant proportion. Due to the increase in the number of surface atoms, insufficient atomic coordination, and high surface energy, these surface atoms exhibit high activity, are extremely unstable, and are readily combined with other atoms.
5)Macroscopic quantum tunneling effect
The ability of microscopic particles to penetrate potential barriers is called the tunneling effect. It’s been discovered that macroscopic quantities, like magnetization intensity of particles and magnetic flux in devices, also exhibit tunneling effects. These are referred to as macroscopic quantum tunneling effects.
2. Precipitation Method
This method involves a salt solution containing one or more soluble cations. When a precipitant (such as OH⁻, CO₃²⁻, SO₄²⁻, or other anions) is added, insoluble hydroxides, carbonates, oxalates, and sulfates are formed and precipitate from the solution. The solvent and the original anions in the solution are then filtered out, and the precipitate is thermally decomposed to obtain the desired oxide powder. When two or more cations precipitate simultaneously in the solution, this process is called co-precipitation.
1)Formation of Precipitation
Common types of precipitation include: coarse crystalline precipitation (e.g., MgNH₄PO₄·6H₂O), fine crystalline precipitation (e.g., BaSO₄), curd-like precipitation (e.g., AgCl), and amorphous precipitation (e.g., Fe₂O₃·xH₂O). The difference between these types primarily lies in the size of the precipitate particles. Crystalline precipitation particles are large, amorphous precipitation particles are small, and curd-like precipitation falls in between. Since the formation of precipitation is a complex process, there is no fully established theory to describe it. The following provides a brief description of the general process of precipitation formation.
2)Formation of Crystal Nuclei
Solute molecules or ions in a supersaturated state, through collision and interaction with each other, aggregate into clusters or molecular groups. When these clusters reach a critical size, they become crystal nuclei—active entities with the maximum free energy for precipitate particle formation. This aggregation and nucleation process is called crystal nucleation. Crystal nucleation is an instantaneous phenomenon, and studying it presents many challenges, meaning its mechanism has not yet been fully resolved. It is generally believed that the crystal nucleus contains no more than 4–8 crystal-forming ions or 2–4 ion pairs.

Conclusion
Nanopowders exhibit unique characteristics that set them apart from both atoms and bulk materials. Their small size leads to distinct physical and chemical properties, such as altered electronic energy levels, quantum size effects, and increased surface energy. These properties result in enhanced reactivity and novel behaviors in various fields, including acoustics, optics, magnetism, and electronics. Additionally, the small size and high surface area of nanoparticles make them particularly valuable in applications where traditional materials fall short. Understanding the behavior and formation processes of nanopowders, such as through methods like co-precipitation, is essential for harnessing their full potential in advanced technological and industrial applications.
Welcome to contact Epic-powder‘s engineers for more information about jet mills that produce fine powders.