This article explores the significant influence of graphite anode material particle distribution on lithium battery performance. It highlights that graphite anodes with an optimal particle size and distribution offer substantial improvements. Such as battery volumetric energy density, charge/discharge efficiency, and cycle stability. The advantages and limitations of different particle sizes are analyzed. As well as the positive effects of a well-structured particle size distribution on electrode-electrolyte permeability and lithium-ion transport dynamics. This provides valuable insights for the design and development of advanced graphite anode materials.

1. Importance of Graphite Anode Material in Lithium-Ion Batteries
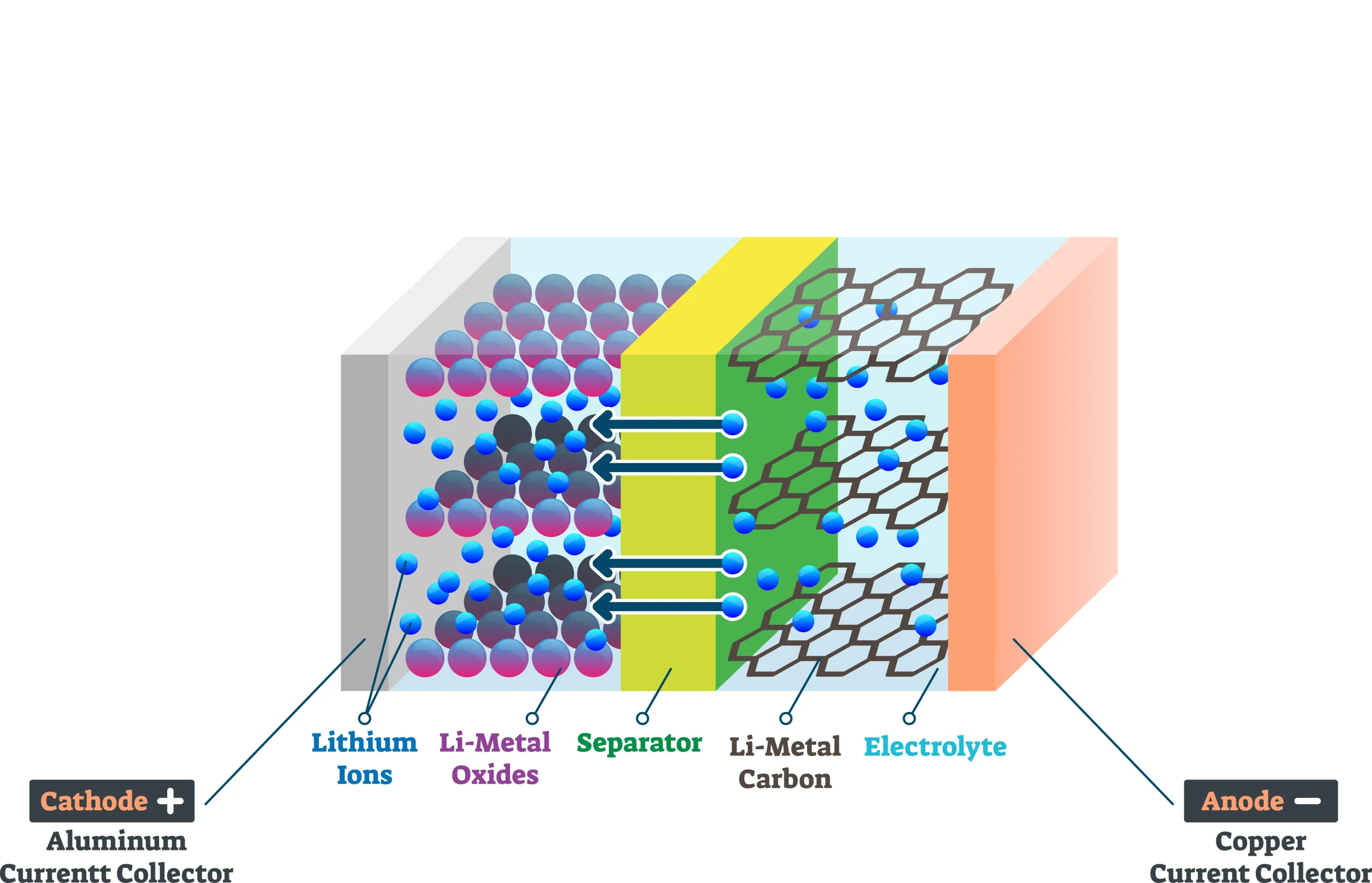
The graphite anode is a key component in lithium-ion batteries, and its particle distribution plays a crucial role in determining overall battery performance. A thorough understanding of the relationship between graphite anode particle distribution and lithium battery behavior is essential for optimizing design and enhancing performance.
2. Effects of Graphite Anode Particle Size on Battery Performance
(I) Advantages and Disadvantages of Smaller Particles
Smaller graphite particles provide a larger specific surface area, which facilitates easier insertion and extraction of lithium ions. This improves initial efficiency and rate performance. For instance, during high-rate charge and discharge cycles, smaller particles can quickly accommodate lithium-ion migration, reducing polarization and boosting the battery’s power output.
However, excessively small particles can also present certain challenges. First, they may lead to increased irreversible capacity. During initial charge and discharge cycles, more lithium ions irreversibly react with the anode’s surface to form a solid electrolyte interphase (SEI) layer, consuming some lithium in the process. Additionally, smaller particles have lower compaction density, which can reduce the volumetric energy density of the battery.
(II) Advantages and Disadvantages of Larger Particles
Larger graphite particles can increase the compaction density, enhancing the battery’s volumetric energy density. However, they also introduce certain drawbacks. The longer diffusion path for lithium ions within large particles can cause uneven formation of the solid electrolyte interphase (SEI) layer during charge and discharge cycles. This irregular SEI layer can thicken over time, increasing internal resistance and accelerating battery capacity decay, ultimately shortening the battery’s cycle life.
(III)Related Testing
A laser particle size analyzer is used to measure particle size distribution by exploiting the scattering effect that particles have on a laser beam. When parallel light encounters particles, some of the light scatters, forming an angle with the main beam. The size of the scattering angle correlates with particle size: larger particles cause smaller scattering angles, while smaller particles result in larger angles. The intensity of the scattered light indicates the quantity of particles of a given size. By measuring scattered light at different angles, the particle size distribution of a sample is determined. Key parameters like D10, D50, and D90 are calculated, representing the particle sizes below which 10%, 50%, and 90% of the particles fall, respectively. These parameters help quantify particle size distribution in terms of volume as well.
3. The Positive Impact of Reasonable Particle Size Distribution on Battery Performance
(I) Enhanced Electrolyte Permeability
A well-optimized particle size distribution improves electrolyte permeability in the electrode. Particles of varying sizes complement each other, forming a complex pore structure that allows the electrolyte to more effectively infiltrate the electrode. This enhances the efficiency of lithium-ion transport and reduces concentration polarization, leading to improved charge and discharge performance.
(II) Optimized Lithium-Ion Transport Kinetics
A balanced particle size distribution also improves lithium-ion transport kinetics. Smaller particles offer more surface area for lithium-ion insertion and extraction, while larger particles provide extended diffusion pathways. This combination helps facilitate smooth lithium-ion movement within the electrode, reducing diffusion resistance and improving both the rate performance and cycle stability of the battery.
(III) Reduced Stress Concentration
During charge and discharge cycles, the insertion and extraction of lithium ions cause volume changes in the electrode, generating stress. A reasonable particle size distribution can minimize stress concentration within the electrode. Different-sized particles undergo varying degrees of volume change, allowing them to buffer one another and reduce mechanical stress on the electrode structure. This mitigates structural damage and prolongs the battery’s cycle life.
4. Conclusion
In summary, the particle size distribution of graphite anode materials plays a crucial role in determining the performance of lithium-ion batteries. Graphite anode materials with optimal particle sizes and well-balanced distribution significantly enhance the battery’s volumetric energy density, charge/discharge efficiency, and cycle stability. Therefore, when designing and developing graphite anode materials, careful consideration of particle size and its distribution is essential. By optimizing process parameters, achieving a balanced particle size distribution can lead to improved overall performance and longevity of lithium batteries.