I. Concept of Layered Oxides
1.1 Structure of Layered Oxides
Layered oxides, with their unique periodic layered structure and two-dimensional ion transport channels, constitute a special class of intercalation compounds. The crystal structure of these materials is characterized by one or more layers of two-dimensional oxide sheets. They are stably connected by ionic or covalent bonds. It is this orderly layered structure that gives layered oxides a large specific surface area. It also provides abundant active sites that facilitate the efficient transmission and rapid reaction of ions and electrons within the material.
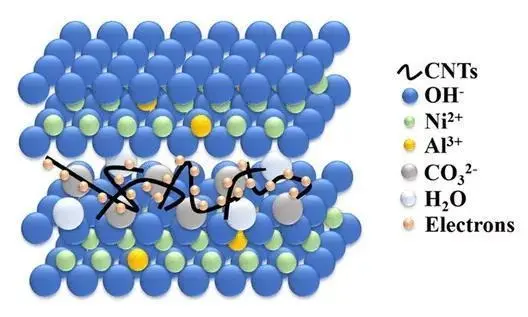
The interlayer spacing of layered oxides is an important characteristic. It can be precisely controlled by adjusting the type or proportion of raw materials used during the preparation process, as well as by varying the temperature, pressure, and other reaction conditions. This control offers the possibility of optimizing the material’s performance. For example, increasing the interlayer spacing can enhance the ion migration rate between layers. It improves the material’s application performance in areas like catalysis and energy storage.
In the field of energy storage, layered oxide materials have been widely studied due to their structural characteristics, particularly in the application of cathode materials for secondary batteries such as lithium-ion, sodium-ion, and potassium-ion batteries. For instance, in lithium-ion batteries, layered oxides like lithium nickel manganese cobalt oxide (NMC) have become the main cathode materials in commercial applications. However, these materials still face challenges, such as interface degradation at high nickel concentrations and high cut-off voltages. Comprehensive imaging and spectroscopy techniques are required to deeply understand their degradation mechanisms and further improve their performance.
The internal structure of layered oxides
The internal structure of layered oxides also plays a significant role in their performance. For example, in lithium-rich layered oxides, advanced microscopy techniques have revealed numerous domains and domain boundaries within the grains. These microstructures significantly impact the migration kinetics of lithium ions, which in turn affects the electrochemical properties of the material.
Layered oxides are not only widely used in energy storage but also exhibit excellent performance in fields such as catalysis and environmental remediation. This is due to their high specific surface area and abundant active sites. It enables them to function as efficient catalysts or adsorbents. By modulating their layered structure, these properties can be further optimized to meet the needs of various applications.
1.2 Properties of Layered Oxides
Layered oxides exhibit a series of attractive physical and chemical properties, making them suitable for a wide range of applications. High stability is a notable feature, enabling them to maintain structural integrity and performance under various conditions. For example, in catalytic reactions, layered oxides can resist chemical corrosion and high-temperature oxidation, maintaining long-term catalytic activity.
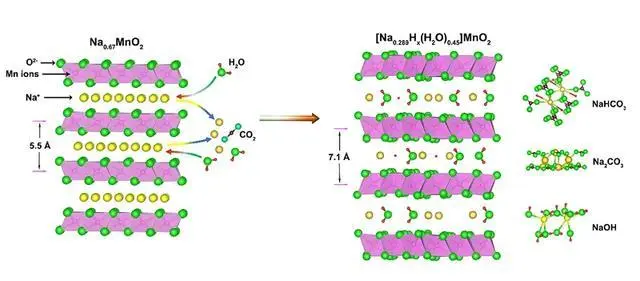
In addition to high stability, layered oxides also possess good electrical conductivity, making them highly suitable for energy storage applications, particularly in batteries and supercapacitors. This conductivity is mainly due to their unique layered structure, which facilitates the rapid transmission of electrons within the material. In lithium-ion batteries, layered oxides are used as cathode materials, and their high conductivity helps to improve the charge/discharge rate and energy density of the battery.
Catalytic activity is another important property of layered oxides. Thanks to their layered structure and abundant active sites. They can be used as catalysts or catalyst supports in various chemical reactions, such as oxidation, reduction, and cracking. Particularly in the catalysis of organic macromolecules and petroleum cracking, layered oxides have demonstrated excellent catalytic performance and selectivity.
Layered oxides also exhibit adsorption and degradation capabilities, which make them valuable in environmental remediation. For example, they can be used to treat heavy metal ions and organic pollutants in wastewater. It helps converting these contaminants into harmless substances through adsorption or degradation. The application of such environmentally friendly materials is of great significance in promoting sustainable development and environmental protection.
1.3 Classification of Layered Oxides
Layered oxides, as a class of materials with unique structures and physicochemical properties, can be classified in various ways, primarily. This is based on their different structures and compositions. Among these classifications, we will focus on several common types of layered oxides. It includes graphene oxide, vanadium oxide, zirconium oxide, layered double hydroxides, and layered oxides used in sodium-ion battery cathode materials.
Graphene Oxide
Graphene oxide, a two-dimensional material composed of a single layer of carbon atoms, has attracted significant attention in recent years. It exhibits extremely high electrical and thermal conductivity. It makes graphene oxide highly promising for applications in electronic devices and energy storage. For instance, in battery technology, the functional groups on the surface of graphene oxide can serve as active sites for chemical modification. It leads to the formation of different active species and providing a multi-electrode material structure.
While lithium-ion batteries with traditional electrode materials have the limitation of a theoretical capacity ceiling, graphene oxide-based composites have demonstrated superior electrochemical properties as both anode and cathode materials. Adding reduced graphene oxide to metal oxides or sulfides in pure oxide or sulfur oxide systems can significantly improve battery performance. Graphene oxide can also be used as a protective coating. This prevents the corrosion of aluminum current collectors in lithium batteries.
Vanadium Oxide and Zirconium Oxide
Vanadium oxide and zirconium oxide are two other important layered oxides. They exhibit excellent photocatalytic properties, making them highly effective for environmental pollution control. Both vanadium oxide and zirconium oxide show outstanding activity and stability in photocatalytic degradation of organic pollutants and in the production of hydrogen through water decomposition.
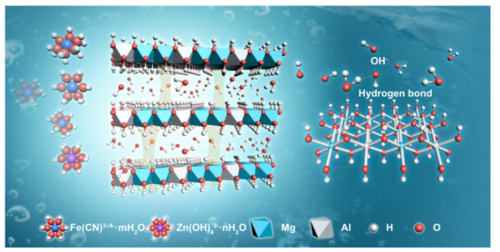
Layered Double Hydroxides (LDHs)
Layered double hydroxides (LDHs) are a class of inorganic materials with characteristic layered structures. Due to their stable two-dimensional lamellar structure, strong self-assembly capabilities, high safety, good biocompatibility, thermal stability, and mechanical properties, LDHs are widely researched for use in drug carrier materials, electrode materials, adsorbents, and other applications.
In recent years, significant progress has been made in the modification of LDHs. Through methods like intercalation, exfoliation, and composites, their performance has been further optimized, broadening their application range.
Other Types of Layered Oxides
There are other types of layered oxides, such as layered transition metal oxides, which also demonstrate unique application value in various fields. The functionalization and electrochemical research of these materials offer strong support for the development of new high-performance batteries, supercapacitors, and other energy storage devices.
2. Application of Layered Oxides
2.1 Catalysis
Layered oxides exhibit great potential in the field of catalysis due to their unique structural characteristics and excellent catalytic performance. Their high specific surface area and abundant active sites make layered oxides ideal candidates as catalysts or catalyst supports, significantly improving the efficiency and selectivity of chemical reactions.
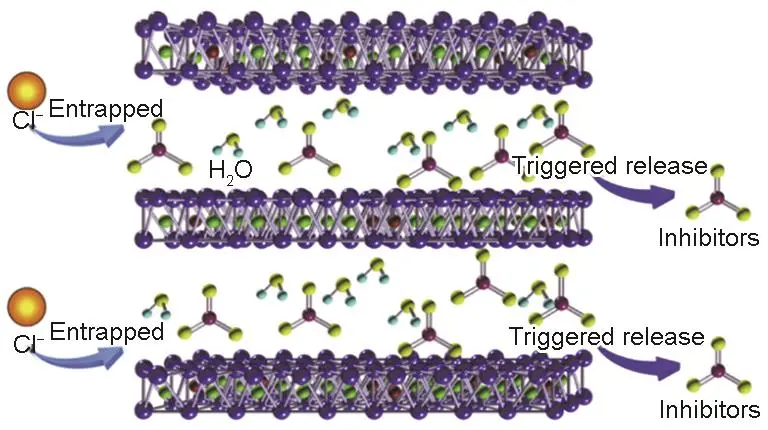
In environmental pollution control, layered oxides play a crucial role. They can serve as highly efficient adsorbents to remove harmful substances from wastewater and exhaust gases through physical or chemical adsorption. At the same time, the catalytic activity of layered oxides can promote the degradation of certain difficult-to-degrade pollutants, converting them into harmless or low-toxic substances. For example, using layered double hydroxide as a catalyst to treat phenol-containing wastewater enables efficient degradation of phenolic compounds.
In energy conversion, layered oxides also show exceptional performance. In hydrogen production through water electrolysis, layered oxides can act as efficient electrocatalysts, reducing the activation energy of the reaction and increasing the hydrogen production rate. Similarly, in fuel cells, layered oxides can serve as catalyst supports for loading precious metal catalysts such as platinum and palladium, thereby enhancing the power generation efficiency and stability of fuel cells.
Layered oxides also have a broad range of applications in organic synthesis. They can function as catalysts in various organic reactions, including oxidation, reduction, and esterification, improving both the yield and selectivity of the reactions. For example, the esterification reaction catalyzed by layered oxides can efficiently convert fatty acids and alcohols under mild conditions, producing ester products with industrial value.
2.2 Energy Storage
In battery technology, layered oxides are widely used in systems such as lithium-ion batteries and sodium-ion batteries. As cathode materials, layered oxides offer high energy density and good cycle stability. For example, in lithium-ion batteries, layered materials such as lithium cobalt oxide, lithium manganese oxide, and lithium iron phosphate have garnered much attention due to their excellent electrochemical properties. These materials provide high specific capacity, good charge and discharge performance, and a stable crystal structure, making lithium-ion batteries ideal for use in electric vehicles, smartphones, and other applications. Similarly, in sodium-ion batteries, layered oxides also demonstrate their advantages as positive electrode materials, supporting the practical application of sodium-ion batteries.
In addition to their role in battery technology, layered oxides are also important in the field of supercapacitors. Supercapacitors play a significant role in energy storage due to their high power density, fast charging and discharging capabilities, and long lifespan. Layered oxides, when used as electrode materials for supercapacitors. It provides abundant active surfaces and fast ion transport channels, enabling efficient charge storage and release. For example, certain transition metal oxides with layered structures are widely used in supercapacitor production. These materials not only offer high specific capacitance but also exhibit excellent cycle stability and rate performance.
2.3 Environmental Governance
Thanks to their unique physical and chemical properties, layered oxides have shown significant advantages and broad application prospects in the field of environmental governance. As industrialization and urbanization continue to advance, environmental pollution has become an increasingly serious issue. Layered oxides have emerged as a powerful tool for addressing these environmental challenges.
In water treatment, layered oxides exhibit excellent adsorption properties. Their layered structure and large specific surface area enable them to effectively adsorb and remove heavy metal ions and organic pollutants from water. For instance, layered double hydroxides (LDHs), which are a class of mixed metal hydroxides with a layered structure, can remove heavy metal ions such as lead, cadmium, and chromium from water through ion exchange or adsorption. Additionally, layered oxides can be modified or combined with other materials to enhance their adsorption capabilities, making them even more effective at addressing water pollution problems.
In air pollution control, layered oxides have demonstrated good performance as catalysts. Certain layered oxides can catalytically oxidize harmful gases such as carbon monoxide and nitrogen oxides in the air at lower temperatures, converting them into harmless carbon dioxide and water. This catalytic effect not only helps reduce air pollutant emissions but also improves air quality.
The crucial role of layered oxides
Layered oxides also play a crucial role in soil remediation. Heavy metal contamination in soil is a significant environmental issue. Layered oxides can remove heavy metal ions from soil through adsorption, ion exchange, and other mechanisms. Furthermore, layered oxides can be combined with microbial remediation techniques to enhance soil cleanup efficiency by promoting the growth and metabolism of microorganisms.
In addition to the aforementioned applications, layered oxides have other potential uses in environmental governance. For example, they can serve as photocatalysts for degrading organic pollutants or as electrode materials for electrochemical wastewater treatment. As research continues to deepen and technology advances, the application of layered oxides in environmental governance will become more widespread and diversified.
3. Challenges Faced
Although layered oxide materials have garnered significant attention in both scientific research and industrial applications. Several challenges remain in their further development and practical use. The primary issue is the complexity of the preparation process and the associated costs. Currently, the synthesis methods for layered oxides—whether chemical precipitation, sol-gel, or hydrothermal synthesis—all involve multiple steps and require precise operations. This not only increases production complexity but also raises the cost of manufacturing the material. For the industry, the key to large-scale production and application of layered oxides lies in simplifying the preparation process, reducing production costs, and ensuring consistent material performance.
Another major challenge is improving the performance stability of layered oxide materials. While these materials demonstrate excellent physical and chemical properties in theory, they may experience performance degradation or even failure in practical applications, especially over extended periods of use. For example, in catalysis, the catalytic activity may gradually decline over time. In energy storage, the capacity and cycle stability of the electrode material may be compromised. Therefore, enhancing the stability of layered oxides and ensuring their long-term performance in real-world applications is a critical area of ongoing research.
Furthermore, environmental adaptability and safety issues must also be addressed. Layered oxides may undergo performance changes or even pose safety risks under specific environmental conditions. Such as high temperatures, high humidity, or corrosive atmospheres. This necessitates thorough consideration of various environmental factors during the material development and design process. This comes hgualong with comprehensive performance evaluation and safety testing.
In summary, while layered oxide materials show great potential, achieving their widespread industrial application requires continuous research and improvements in several areas. It includes preparation methods, performance stability, and environmental adaptability.
For more information about powder processing machines, please feel free to contact Epic Powder‘s professional team.